Summary
Steven E. Jones and Jeff Farrer, both among the authors of the 2009 paper "Active Thermitic Material..." (ATM, [1]) by Harrit e.al., have now confirmed four claims I have made about that paper and the red-gray chips discussed therein:
- The chips contain traces of strontium and chromium, corroborating my claim that some chips may be LaClede steel primer, which contains ca. 1% by weight Strontium Chromate pigments [2]
- Their XEDS data of chips a-d is consistent with kaolin as the sole ingredient that contains Al
- They concede that a DSC experiment, done by LLNL scientists Tom Tillotson and Alex Gash and refered to in ATM, may have been performed under inert atmosphere and not, as they previously believed, under air
- Red-gray chips found in WTC are not all the same material, they represent at least two different materials
Jones and Farrer speak
A few days ago, on september 8th, Steven Jones posted recommendations to an unnamed scientist who wants to do a replication of the Harrit e.al. experiments on red-gray chips at 911Blogger [3]. A day later, he appended comments (or paraphrases thereof) that he had received from Jeff Farrer. Please find the bulk of his post quoted below.
I will now show how Jones and Farrer confirm four claims I have made about their experiments on red-gray all along.
Discussion
1. Farrer corroborates Strontium Chromate from LaClede primer paint
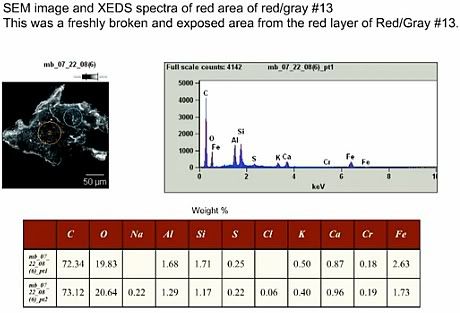
As I have previously shown [2], the four red-gray chips labeled "a-d" in Figures 2 - 11 of ATM [1] are consistent with what one would expect from chips of LaClede Standard Primer - a red paint that, according to NIST documentation, was painted on the floor joists of the WTC twin towers. These floor joists probably had more painted surface than the perimeter columns (painted with Tnemec 69 or 99) and the core columns (painted with unknown primer(s)) and can thus be expected to be abundant in WTC dust.
To recap: LaClede standard primer was a paint that, per specification, consisted of 71.5% by weight epoxy (organic matrix) and 28.5% mineral pigments. Of the pigments, 55% by weight was red iron oxide (i.e. hematite = Fe2O3 with a size most likely in the range 100-300 nanometers), 41% aluminium silicates (with kaolin, a naturally mined clay silcate, chemical sum formula Al2Si2O9H5, being the most mundane candidate) and 4% Strontium Chromate (SrCrO4). This would be equivalent to there being approximately 0.5% Strontium and 0.3% Chromium in the ready paint, along with 11% iron and 2.5% and 2.4% silicon and aluminium. I have further shown that an XEDS spectrum of this paint at 20 keV (the electron energy used by Harrit e.al. for their Fig. 7) would show a small signal for Cr, but that Sr would quite likely be missed due to its small signal being right underneath the much larger Si-signel. I have pointed to a letter by Niels Harrit [4] in which Harrit documented that small signals for both Cr and Sr were detectable at least in their chip a.
We learn via Steven Jones now that Jeff Farrer has done further TEM-studies on red-gray chips, and has confirmed the presence of both Strontium and Chromium:
6. Jeff notes that in his TEM analyses he observed “very small (nanometer-scale) Pb particles in the TEM samples” as well as strontium and chromium in small amounts. (Much of the TEM analysis was performed at higher magnification than used in the SEM analysis done in the paper.) Thus, red/gray chips which match ours will show these same elements under TEM analysis.
TEM is "Transmission electron microscopy". The XEDS spectra shown in ATM[1] were all derived from "SEM" equipment, that is "Scanning electron microscope". Jones is clear that Farrer did identify Sr and Cr with the TEM.
This would corroborate the result from [4], that some red-gray chips contain particles (pigments) with Strontium and Chromium - a result clearly consistent with my assertion that some chips are LaClede primer.
It is true that Dr. Millette [8] did not show any Sr in his TEM data. I submit that this could possibly result from Sr-chromate making up only 4% of the LaClede pigment - one part in 25. It isn't totally unlikely that a microscopic LaClede sample with countably few pigments by chance simply contains no Sr-chromate pigment. I wish though Dr. Millette would go back to the lab and specifically search for such pigments (they should be recognizable by their probable shape, which is typically acicular, i.e. needle-shaped, with length in the range of 1-4 micrometers)
It would be interesting to know how Farrer identified elements on the TEM, and see his actual results (images, spectra...). One technique available on TEM to identify not elements as such but crystal structures and thus, potentially, the minerals in question, is TEM-SAED ("Selected area electron diffraction"). I wonder if Farrer has specifically identified Strontium Chromate. I call upon Drs Farrer and Jones to publish their TEM data as soon as possible!
2. Farrer confirms Al and Si consistent with kaolin
In his own initial post, Jones makes the following assertion:
... looking at aluminum-containing platelets which we were able to isolate quite well in the thin sample. We found that the Al and Si are in fact NOT in equal amounts; the Al:Si ratio came out to approximately 0.92 (based on atomic wt %, TEM focused on a platelet.) How could this be the mineral kaolinite as you suggest, for which the Al:Si ratio is exactly 1.0? Formula: Al2Si2O5(OH)4
An Al:Si ratio of 0.92 would mean there is more silicon than aluminium in those "aluminum-containing platelets" - a strange finding for one who claims to have a formulation of Fe-Al-thermite in which the Al is found in said platelets. Note that Jones fails to mention that the same platelets also contain a very significant amount of oxigen, as can be seen in Fig. 11a of [1]. It would be interesting to get a value for the Al:O ratio there!
However, Jones's claim that the measured ratio of 0.92 disproves kaolinite, is FALSE, as Farrer has pointed out. Jones added the following remark a day later:
5. With regard to the 0.92 ratio, Jeff notes that he did not use standards for the TEM/XEDS analysis so this ratio could be consistent with unity. The interested scientist is encouraged to use standards for the TEM/XEDS so this ratio can be pinned down definitively.
Kudos to Farrer for pointing this out, and to Jones for faithfully forwarding that comment. So it seems Farrer's TEM-data on those platelets is consistent with kaolin after all!
(To wrap up my argument: Fig. 7 (XEDS spectra for bulk or red layers) shows that chips a-d all have Al:Si ratios near unity. The only constituent of chips a-d identified by the Jones team as containing Al are these platelets, which means that all, or almost all, the Al in the chips is actually contained in the platelets. The elemental composition as well as the morphology of these platelets is entirely consistent with kaolin clay, a common paint ingredient. It is indeed the best explanation for ALL the data Harrit e.al. have presented on chips a-d. So if the Al in the platelets is present in a stoichiometric proportion to Si to form kaolin, or even sllightly too little Al, then there is no Al left - neither in the platelets nor elsewhere in the chip - to account for a hypothetical presence of elemental Al.)
3. Farrer concedes he may have done DSC-test the wrong way
The context for that topic is this: In ATM [1], Harrit e.al. present, in Fig. 19, DSC traces for four (unknown, uncharacterised) chips. They compare one of these with a DSC trace taken from a paper by LLNL scientists Tom Tillotson and Alex Gash [5], which was from a sample of experimental nano-thermite. Harrot e.al. claim that the DSC traces basically have the same characteristics (while in fact it can be clearly seen that they are quite different in many respects), and take this as one of their best pieces of evidence that the red-gray chips are not thermitic. The DSC test on the red-gray chips was done by Jeff Farrer, and it was done under an atmosphere of normal air, i.e. in the presence of ca. 21% oxygen. In Tillotson e.al., no indication is given if the test was done under air or inert gas.
Steven Jones and Niels Harrit have claimed on several occasions that Jeff Farrer contacted Tillotson and Gash before doing his DSC test and learned from them that they used air. I have called this a lie. It is patently obvious why doing the experiment under air would be a fool's errand when you know (as both Tillotson e.al. and Harrit e.al. did) that your sample contains organic material which is likely to combust under air when heated. I just didn't know if the lie orignated with Farrer, who is alleged to have called the LLNL scientists, or with Jones or Harrit, who may have invented that bit of information about Farrer. I know however of two emails that Gash and Tillotson wrote on the matter and that prove Jones and Harrit wrong [6]. First from Gash's email:
[...] As you correctly point out DSC in an O2 atmosphere will combust the organic impurities and greatly add to the energy release. However the DSC in question was done in ultra pure nitrogen. [...]
Alex
Then Tillotson:
The experiment was performed as Alex described...in ultra pure nitrogen as is standard technique here at LLNL. If Mr. Farrer did contact me I can guarantee you that I did not respond to his questions.
Tom Tillotson
Now, Jeff Farrer has advised Jones to backpaddle from the earlier claims. Jones appends Farrer's comment (my emphasize):
1. Dr. Farrer contacted Dr. Tillotson of LLNL regarding the LLNL production and ignition of nano-thermite; Dr Tillotson said the experiments were likely done in atmosphere. After publication of our paper, others have suggested that the experiments in the LLNL publication were performed in an inert atmosphere; so the picture is not clear to us at this time and further contact with the LLNL scientists is advised. It would be best to run studies in both atmosphere and in an inert gas.
Good to see that all of a sudden they really don't know. It must be pointed out that Tillotson has expressedly denied the claim that he replied to a question by Farrer, if ever he received one.
Obviously, it should be assumed at this time that
- Tillotson and Gash did their experiment under inert gas
- Farrer did his test under 21% oxygen
- The results of both teams can thus not be compared
- No DSC test will yield any useful results with regard to identifying or excluding thermite if done under air
- A replication of the DSC is thus not desirable and should be advised against
To pile up, Alex Gash today believes DSC is not a good method at all to characterize a (nano-)thermite reaction. In his email, he continues:
While that may or may not be the case over the years we have come to rely less and less on the enthalpy from DSC for irreversible reactions as an absolutely accurate value.
In irreversible high energy processes the solid is undergoing many changes that may lead to inefficient heat transfer tot he DSC sensor and thus an inaccurate heat flow measurement. At the time of publication, we had more faith in the absolute value of these measurements. That is not to say DSC is not useful, quite the contrary. It gives us a reasonable idea how energetic a composition may be, it identifies decomposition temperatures, and is very accurate for determining the enthalpy of reversible heat flow (e.g., phase transitions, melting etc..). Since the publication of that paper we have found that combustion calorimetry is a far more accurate way to determine reaction enthalpy.
Gash confirms what any person experienced with and knowledgable about DSC could have told you: DSC is very good for physical processes, good for decomposition, but not good for vigorous chemical reactions. Another excellent reason why replication of Farrer's DSC-expermient is not a good idea.
4. Jones admits that red-gray chips may be from different materials
In a previous post, I have already explained why red-gray chips aren't all the same [7]: Harrit e.al. themselves point out how different chips are characterized by different significant elements. It really is quite obvious.
Yet, Jones and Harrit go on and pretend like all chips are basically the same material. In particular, their MEK-soaked chip (Fig. 12-18) is obviously quite different from chips a-d (Fig. 2-11, which are much more likely all the same stuff) in several respects, yet they pretend that a result found on that MEK-soaked chip (apparent trace occurance of elemental Al) can be extrapolated to chips a-d. Further on, no spectra or images are provided for the four chips burned in the DSC (Fig. 19), so their identity and characteristics remain unknown - it is simply assumed, without argument, that they are the same material.
It can't be stressed enough that all conclusions of ATM [1] are fundamentally dependent on the unproven, and actually refuted, assumption that all red-gray chips in WTC dust that are attrected to a magnet are basically the same (thermitic) material because they lump together results gained from different specimens and form a conclusion that is assumed to be valid for all of them.
With his new statement, Steven Jones now implies that red-gray chips extracted from WTC dust by magnet are from different materials. He writes:
I (Dr. Jones) have searched Millette's plots and see no indication of strontium (Sr) or lead (Pb) in his samples, but he does report titanium (Ti) which we do not see. Thus, his samples do not appear to be the same material as what we reported on.
Please keep in mind that Millette used the very same method to gather red-gray chips that Jones did. Thus, if Millette can extract a different material, so can Jones, and with that reasoning, he has to consider the possibility that, for example, the MEK-soaked chip wasn't the same material as chips a-d, or that the chips Farrer wasted in the DSC were different from each other, from chips a-d, and / or different from the MEK-soaked chip.
Also, note how Jones determines that "his [Millette's] samples do not appear to be the same material": he notes that some of Millettes chips, according to SEM-XEDS spectra, contain element X and not Y, while some of Jones's chips contain Y and not X. The same argument can be applied to Jones's own chips:
- The MEK-soaked chip contains Zn and Mg but no Na or K; Chip (c) on the other hand contains neither Zn nor Mg, but does show Na and K.
- The DSC-residue of a chip shown in Fig. 25 shows Ti; Neither chips a-d nor the MEK-soaked chip have Ti.
- The chip in Fig. 31 contains Pb. No other chip shown in [1] contains Pb
- The gray layer of the chip in Fig. 33 contains no Fe; all gray layers in Fig 6 are dominated by Fe
I call on Steven Jones to puclicly acknowledge that obviously the red-gray chips are of different materials, rendering the conclusions of Harrit e.al. invalid!
There's even more: Jones rejects Millette's samples on the ground that Millette reports no Pb, Sr and Cr. By the same reasoning, Jones ought to have rejected ALL specimens presented in his own paper, ATM, on the grounds that none are shown to contain Pb, Sr and Cr!
He alleges that Millette's finding of Ti is grounds to exclude the specimen from the study, yet included a specimen with Ti in ATM.
The explanations for these discrepancies are obvious:
- As the chips are obviously from different materials, some are bound to contain Pb, others not; some are bound to contain Sr and Cr, others not; some are bound to contain Ti, others not; some are bound to contain Zn or Mg, others not; etc.
- On each specimen, it would be possible and easy to miss a small trace of an element when scanning the bulk of a particle with SEM-EDS, but finding small particles containing that element within the specimen when focussing the much hiigher resolution of TEM-EDS on select pigments
Farrer's finding of particles containing Pb, Sr and Cr is very interesting, but near useless without having the actual data for reference. I call upon Dr. Farrer to publish his TEM-data fully and as soon as possible!
Conclusions
Steven Jones did not intend this, isn't perhaps aware of it and would probably deny it, but his latest comments have strengthened the hypothesis that some of the red-gray chips were corroded steel chipped off the WTC floor joists, which were painted by LaClede Steel Company with a primer containing pigments of Iron Oxide and Kaolin along with traces of Strontium Chromate, by confirming that
- some chips contain particles with strontium and chromium
- the Al:Si ratio observed in many of the chips is consistent with Kaolin
Further, he has retracted the claim that DSC tests ought to be done under air to compare the results with actual nano-thermite. This puts into further doubt the validity and usefulness of doing DSC tests on the red-gray chips and speaks against repeating such tests.
Lastly, Jones has admitted, by implication, that the WTC dust contains several different materials that form red-gray, magnetically attracted chips. He has provided criteria for when to doubt that two chips are of the same material. Applying the same criteria to ATM (Harrit e.al., [1]) invalidates at once all major conclusions of that paper and resets the status of the debate to "there is no evidence that any red-gray chips contain aluminothermic material".
The Source: Recent remarks by Steven Jones at 911Blogger
Quoting at length from the post for posterity - first what Jones originally posted (I left out parts that bring up other issues than the four I discuss here):
Dear [Interested Scientist],
Yes, I would encourage you to do a follow-up study on the World Trade Center dust, after you have carefully read our “Active Thermitic Materials...” paper. [Niels Harrit, Jeffrey Farrer, Steven Jones, et al. "Active Thermitic Material Discovered in Dust from the 9/11 World Trade Center Catastrophe", THE OPEN CHEMICAL PHYSICS JOURNAL, April 2009.]
Among the most salient observations in that paper are these:
1. the observation of elemental-iron-rich spheres in the ash following ignition of the red/gray chips in the Differential Scanning Calorimeter (DSC),
2. the sharp peaking of the heat-traces in each case for the ignition of red/gray chips in the DSC (Figure 19).
Therefore, I am pleased that you propose to do DSC analyses along the lines that we preformed; as you noted, James Millette did NOT do DSC analyses at all for his report MVA9119. What a shame, really, and I hope you will do better as you propose.
[...]
When Dr Farrer burned epoxy paint in the DSC, it gave a very broad thermal trace, NOT at all like the spiked exothermic DSC peak in our Fig 19. This is one of the many tests he did to check things.
[...]
You suggest that you would like to ignite the red material in an inert atmosphere, which is not a bad idea but there are caveats. Dr Farrer of our team contacted one of the LLNL scientists about this issue, and was informed that the LLNL tests of nano-thermite were performed in air; which is why we did our tests in air also. Thus, we could make direct comparisons with the LLNL data on nano-thermite fabricated at the LLNL laboratory.
Later, we mixed up some ultra-fine aluminum and iron-oxide powders thus making a type of nano-thermite (but with no organic matrix). This was run in the DSC at BYU in an inert atmosphere up to 700C – and it did not ignite! We concluded that oxygen may be important to get the reaction initiated.
You say that the exothermic peaks we observed in the DSC (our Figure 19) could be due to burning of epoxy paint. Not according to our experiments -- that is, when Dr Farrer burned epoxy paint in the DSC, it gave a very broad thermal trace, NOT at all like the spiked exothermic DSC peaks in Fig 19. Igniting paint in the same DSC is one of many tests performed to double-check our experiments, and I urge you to do similar tests.
Please keep these facts in mind as you undertake DSC studies – which I welcome! Yes, I was surprised that James Millette did not even perform DSC studies.
[...]
Dr Farrer and I did some work with Transmission Electron Microscopy after the paper was published, looking at aluminum-containing platelets which we were able to isolate quite well in the thin sample. We found that the Al and Si are in fact NOT in equal amounts; the Al:Si ratio came out to approximately 0.92 (based on atomic wt %, TEM focused on a platelet.) How could this be the mineral kaolinite as you suggest, for which the Al:Si ratio is exactly 1.0? Formula: Al2Si2O5(OH)4 .
The accuracy of the TEM analysis should allow you (and Millette) to determine if you are indeed looking at the same material that we reported on, beginning with the Al:Si ratio.
I encourage you to do TEM analysis as we have done. Studying electron-diffraction patterns obtained with the TEM, Dr. Farrer found that that the iron-oxide was in the form Fe2O3. He did not see a pattern demonstrating that aluminum was in a form he recognized by this method, which surprised us. There are possible explanations for this; see for example http://www.tms.org/pubs/journals/jom/0203/perepezko-0203.html . I'll leave it at that for now. I have encouraged Dr. Farrer to write up and publish his TEM findings. Did Millette see an electron diffraction pattern demonstrating that aluminum occurs in the form of kaolinite? His report does state: Millette report: "TEM-SAED-EDS analysis of a thin section of the red layer showed equant-shaped particles of iron consistent with iron oxide pigments and plates of kaolin clay (Figures 20 and 21). The matrix material of the red coating layer was carbon-based. Small numbers of titanium oxide particles consistent with titanium dioxide pigment and some calcium particles were also found (Appendix F).” We did TEM analysis also, years ago now, but we did not see any titanium in the red/gray chips! (Referring specifically to the clean-surface chips; see Figs. 6 and 7 in our published paper.) More and more, it appears that Millette was simply not looking at the same material that we studied. Why would he not measure the electrical resistivity of his red material (discussed in our paper) right off? That's what gets me – he could have saved himself a lot of time. Finally he gets to TEM analysis, and finds that he has titanium oxide! How can he claim its the same material? What a waste of time. I hope you will not make the same mistake. Sincerely, Steven E. Jones
A day later, SE Jones had received feed-back from his collaborater at BYU, Jeff Farrer (manager of the BYU TEM-lab), and appended his blog post with the following clarifications (again, only showing the parts that are of interest here):
Note added, based on comments received 9-9-12 from Dr. Jeffrey Farrer.
1. Dr. Farrer contacted Dr. Tillotson of LLNL regarding the LLNL production and ignition of nano-thermite; Dr Tillotson said the experiments were likely done in atmosphere. After publication of our paper, others have suggested that the experiments in the LLNL publication were performed in an inert atmosphere; so the picture is not clear to us at this time and further contact with the LLNL scientists is advised. It would be best to run studies in both atmosphere and in an inert gas. 2. The DSC run with the ultra-fine aluminum and iron-oxide (which did not ignite in atmosphere) may have been heated to approximately 800 degrees centigrade. Jeff will check his notes.
[...]
5. With regard to the 0.92 ratio, Jeff notes that he did not use standards for the TEM/XEDS analysis so this ratio could be consistent with unity. The interested scientist is encouraged to use standards for the TEM/XEDS so this ratio can be pinned down definitively.
6. Jeff notes that in his TEM analyses he observed “very small (nanometer-scale) Pb particles in the TEM samples” as well as strontium and chromium in small amounts. (Much of the TEM analysis was performed at higher magnification than used in the SEM analysis done in the paper.) Thus, red/gray chips which match ours will show these same elements under TEM analysis.
I (Dr. Jones) have searched Millette's plots and see no indication of strontium (Sr) or lead (Pb) in his samples, but he does report titanium (Ti) which we do not see. Thus, his samples do not appear to be the same material as what we reported on.
References
[1] Niels H. Harrit, Jeffrey Farrer, Steven E. Jones, Kevin R. Ryan, Frank M. Legge, Daniel Farnsworth, Gregg Roberts, James R. Gourley and Bradley R. Larsen: Active Thermitic Material Discovered in Dust from the 9/11 World Trade Center Catastrophe. The Open Chemical Physics Journal, 2009, 2, 7-31
[2] Oystein: Another primer at the WTC: LaClede Standard Primer. 2012/03/16
[3] Steven E. Jones: Letter regarding red/gray chip analyses. Blog post at 911Blogger, 2012/09/08. Last retrieved 2012/09/12.
[4] Niels H. Harrit: Why The Red/Gray Chips Are Not Primer Paint. Open Letter, May 2009
[5] T.M. Tillotson et al: Nanostructured energetic materials using sol-gel methodologies. Journal of Non-Crystalline Solids 285 (2001) 338-345
[6] T.M. Tillotson and Alexander Gash: E-Mails. As quoted by "Moorea" at the JREF forum on 2012/09/09. Original mails probably written on or shortly before either 2010/07/12 or 2010/12/07.
[7] Oystein: Why red-gray chips aren't all the same. 2012/03/14
[8] James R. Millette: Revised Report of Results: MVA9119. Progress Report on the Analysis of Red/Gray Chips in WTC dust. Prepared for Classical Guide, Denver, 01 March 2012.